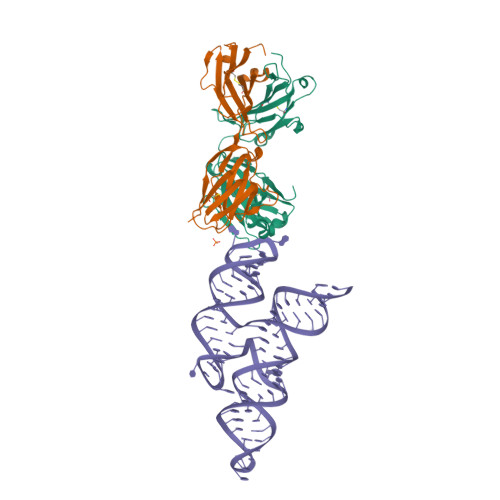
Crystal structure of a highly conserved enteroviral 5' cloverleaf RNA replication element.
Publication Type:
Journal ArticleSource:
Nat Commun, Volume 14, Issue 1, p.1955 (2023)Keywords:
Nucleic Acid Conformation, Phylogeny, Poliovirus, Protein Binding, RNA, RNA Replication, RNA, Viral, Virus ReplicationAbstract:
<p>The extreme 5'-end of the enterovirus RNA genome contains a conserved cloverleaf-like domain that recruits 3CD and PCBP proteins required for initiating genome replication. Here, we report the crystal structure at 1.9 Å resolution of this domain from the CVB3 genome in complex with an antibody chaperone. The RNA folds into an antiparallel H-type four-way junction comprising four subdomains with co-axially stacked sA-sD and sB-sC helices. Long-range interactions between a conserved A40 in the sC-loop and Py-Py helix within the sD subdomain organize near-parallel orientations of the sA-sB and sC-sD helices. Our NMR studies confirm that these long-range interactions occur in solution and without the chaperone. The phylogenetic analyses indicate that our crystal structure represents a conserved architecture of enteroviral cloverleaf-like domains, including the A40 and Py-Py interactions. The protein binding studies further suggest that the H-shape architecture provides a ready-made platform to recruit 3CD and PCBP2 for viral replication.</p>