Molecular basis of hemoglobin binding and heme removal in Corynebacterium diphtheriae.
Publication Type:
Journal ArticleSource:
Proc Natl Acad Sci U S A, Volume 122, Issue 1, p.e2411833122 (2025)Keywords:
Bacterial Proteins, Carrier Proteins, Corynebacterium diphtheriae, Crystallography, X-Ray, Heme, Hemoglobins, Humans, Iron, Models, Molecular, Protein BindingAbstract:
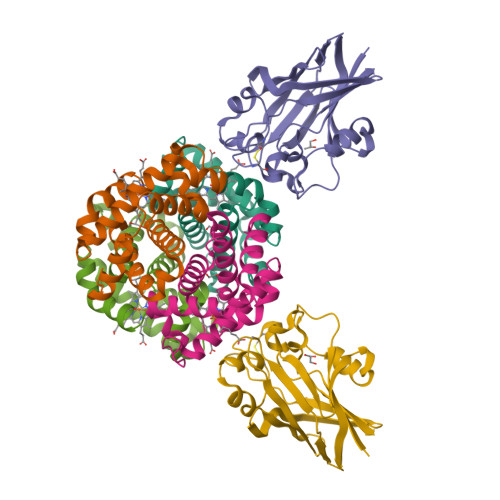
<p>To successfully mount infections, nearly all bacterial pathogens must acquire iron, a key metal cofactor that primarily resides within human hemoglobin. causes the life-threatening respiratory disease diphtheria and captures hemoglobin for iron scavenging using the surface-displayed receptor HbpA. Here, we show using X-ray crystallography, NMR, and in situ binding measurements that selectively captures iron-loaded hemoglobin by partially ensconcing the heme molecules of its α subunits. Quantitative growth and heme release measurements are compatible with acquiring heme passively released from hemoglobin's β subunits. We propose a model in which HbpA and heme-binding receptors collectively function on the surface to capture hemoglobin and its spontaneously released heme. Acquisition mechanisms that exploit the propensity of hemoglobin's β subunit to release heme likely represent a common strategy used by bacterial pathogens to obtain iron during infections.</p>