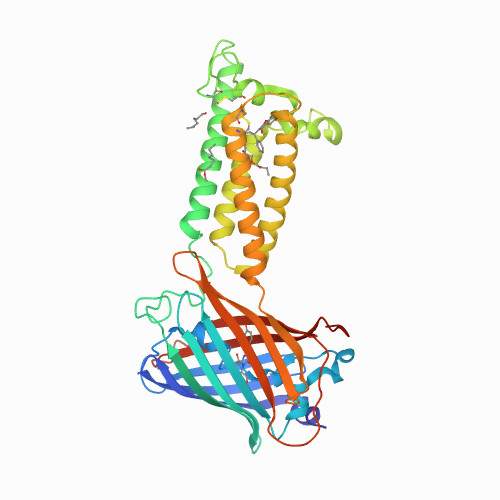
Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation.
Publication Type:
Journal ArticleSource:
Science (2020)Abstract:
<p>Vitamin K antagonists are widely used anticoagulants targeting vitamin K epoxide reductases (VKOR), a family of integral membrane enzymes. To elucidate their catalytic cycle and inhibitory mechanism, here we report eleven x-ray crystal structures of human VKOR and pufferfish VKOR-like with substrates and antagonists in different redox states. Substrates entering the active site in a partially oxidized state form a cysteine adduct that induces an open-to-closed conformational change, triggering reduction. Binding and catalysis is facilitated by hydrogen-bonding interactions in a hydrophobic pocket. The antagonists bind specifically to the same hydrogen-bonding residues and induce a similar closed conformation. Thus, vitamin K antagonists act through mimicking the key interactions and conformational changes required for the VKOR catalytic cycle.</p>